Pregabalin CAS 148553-50-8 Purity >99.0% (HPLC) Antiepileptic API
Manufacturer with High Purity and Commercial Production
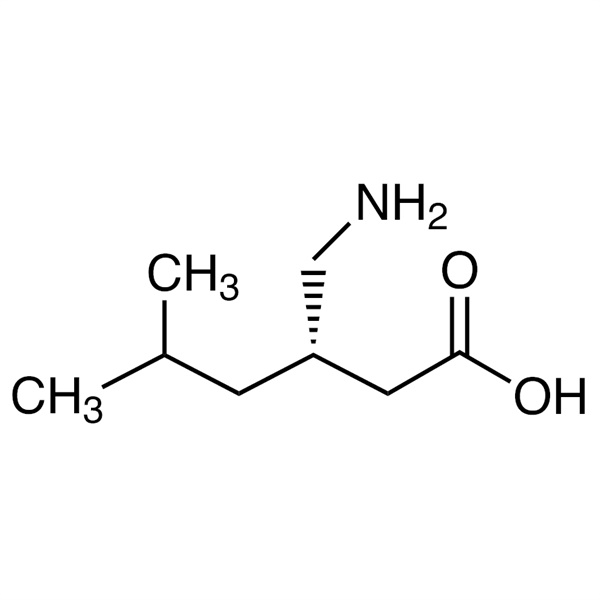
Chemical Name: Pregabalin CAS: 148553-50-8
Pregabalin is a second-generation antiepileptic drug (AED)
| Chemical Name | Pregabalin |
| Synonyms | (S)-3-(Aminomethyl)-5-Methylhexanoic Acid; PD-144723; PD144723; CI 1008; CI-1008; Lyrica |
| CAS Number | 148553-50-8 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H17NO2 |
| Molecular Weight | 159.23 |
| Melting Point | 194.0~196.0℃ |
| Density | 0.997±0.06 g/cm3 |
| Solubility in Water | Practically Insoluble in Water |
| Solubility | Very Slightly Soluble in Ethanol |
| Shipping Condition | Under Ambient Temperature |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification | IR: Similar to Reference Substance |
| Specific Rotation | +10.0°~+13.0° (C=1, Water) |
| Loss on Drying | <0.50% |
| Sulphated Ash | <0.10% |
| Related Substances | Impurity A <0.15% |
| Unspecified Impurities Eluting Before Pregabalin (test A) <0.10% | |
| Unspecified Impurities Eluting After Pregabalin (test B ) <0.10% | |
| Total for test A and B <0.50% | |
| Purity By HPLC R-Isomer | <0.15% |
| Heavy Metals (as Pb) | <10ppm |
| Chlorides (Cl) | <0.05% |
| Residual Solvents | |
| Isopropyl Alcohol | <5000ppm |
| Ethyl Acetate | <5000ppm |
| Tetrahydrofuran | <250ppm |
| Isopropyl Ether | <500ppm |
| Ethanol | <1000ppm |
| Purity / Analysis Method | >99.0% (HPLC on dried basis) |
| Test Standard | Enterprise Standard |
| Usage | Antiepileptic Drug API |
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light & moisture

Risk Codes R63 - Possible risk of harm to the unborn child
R48/22 - Harmful danger of serious damage to health by prolonged exposure if swallowed.
R39/23/24/25 -
R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed.
R11 - Highly Flammable
Safety Description S22 - Do not breathe dust.
S36/37 - Wear suitable protective clothing and gloves.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S16 - Keep away from sources of ignition.
S7 - Keep container tightly closed.
UN IDs UN1230 - class 3 - PG 2 - Methanol, solution
WGK Germany 3
Pregabalin is a second-generation antiepileptic drug (AED) known with the proprietary brand name of Lyrica (Pfizer, Tadworth) in the UK and USA, having a γ-amino butyric acid structure on its molecular structure, which has anticonvulsant effects, and is successfully developed by the company Pfizer for the treatment of peripheral neuropathic pain, or adjuvant treatment of partial seizures. Pregabalin is a Anticonvulsant and antiepileptic drug used to treat epilepsy, neuropathic pain, fibromyalgia, and generalized anxiety disorder. Its use for epilepsy is as an add-on therapy for partial seizures with or without secondary generalization in adults. In December 2008, the US Food and Drug Administration (FDA) approved pregabalin (trade name "Lyrica") for the treatment of diabetic peripheral neuropathic pain (DPN) and postherpetic neuralgia (PHN)which are Both the most common neuropathic pains. Pregabalin has an approved indication and is widely used for the treatment of generalized anxiety disorder. Several randomized, double- blind, placebocontrolled trials found that pregabalin is an effective treatment for patients with generalized anxiety disorder and social anxiety disorder. Dose titration Epilepsy-adjunctive therapy: 25 mg bd for 7 days, to be increased by 50 mg every 7days; usual maintenance 300 mg daily, divided into 2 or 3 doses (max. 600 mg daily, divided into 2 or 3 doses). Generalized anxiety disorder: 150 mg daily, divided into 2 or 3 doses, for 7 days, to be increased by 150 mg every 7 days (max. 600 mg daily, divided into 2 or 3 doses). If stopping pregabalin, it is recommended to taper over at least 1 week to avoid abrupt withdrawal. Cautions Patients with conditions that may precipitate encephalopathy. Patients with severe congestive heart failure.