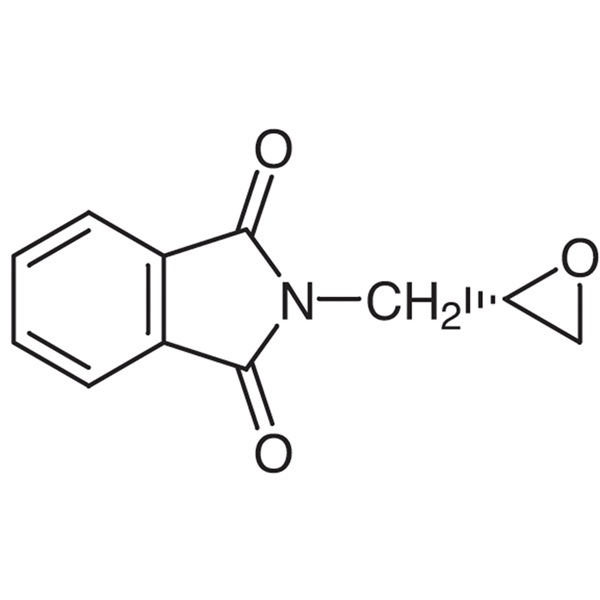
(S)-(+)-Glycidyl Phthalimide CAS 161596-47-0 Purity ≥98.0% (HPLC) Rivaroxaban Intermediate Factory
Manufacturer Supply Rivaroxaban Related Intermediates:
(S)-(+)-Glycidyl Phthalimide CAS 161596-47-0
4-(4-Aminophenyl)morpholin-3-one CAS 438056-69-0
Rivaroxaban Intermediate CAS 446292-07-5
5-Chlorothiophene-2-Carboxylic Acid CAS 24065-33-6
5-Chlorothiophene-2-Carbonyl Chloride CAS 42518-98-9
Rivaroxaban API CAS 366789-02-8
| Chemical Name | (S)-(+)-Glycidyl Phthalimide |
| Synonyms | (S)-Glycidyl Phthalimide; (S)-N-Glycidylphthalimide; (S)-N-(2,3-Epoxypropyl)phthalimide; Rivaroxaban Intermediate; Rivaroxaban Impurity 39; N-(S)-Glycidylphthalimide |
| CAS Number | 161596-47-0 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H9NO3 |
| Molecular Weight | 203.19 |
| Melting Point | 100.0 to 104.0℃ |
| Density | 1.446±0.06 g/cm3 |
| Sensitive | Moisture Sensitive |
| Solubility | Soluble in Chloroform |
| Storage Condition | Under Inert Gas (Nitrogen or Argon) at 2~8℃ |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification | HPLC |
| Loss on Drying | ≤0.50% |
| Sulphate Ash | ≤0.50% |
| Purity / Analysis Method | ≥98.0% (HPLC) |
| Phthalimide | ≤0.10% |
| Single Max Impurity | ≤0.50% |
| Total Impurities | ≤2.0% |
| Epichlorohydrin | ≤0.10% |
| Glycidol | ≤0.10% |
| Chiral Purity | R-isomer ≤2.0% (HPLC) |
| Test Standard | Enterprise Standard |
| Usage | Rivaroxaban (CAS: 366789-02-8) Intermediate |
(S)-(+)-Glycidyl Phthalimide CAS: 161596-47-0 Synthesis Route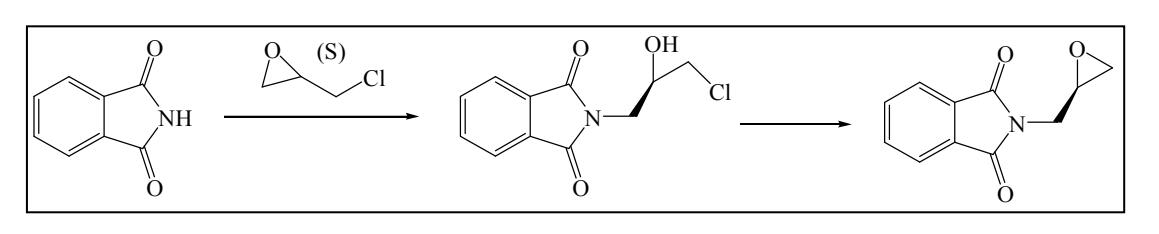


Hazard Symbols Xi - Irritant
Risk Codes
41 - Risk of serious damage to eyes
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S39 - Wear eye / face protection.
WGK Germany 2
HS Code 2925190090
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (S)-(+)-Glycidyl Phthalimide (CAS: 161596-47-0) with high quality. It is an intermediate typically in the synthesis of Rivaroxaban (CAS: 366789-02-8), in the treatment of Venous Thromboembolism (VTE).
Rivaroxaban (Xarelto; CAS: 366789-02-8) is an antithrombotic drug and was developed in a collaboration between the German Bayer Pharmaceuticals and American Johnson company. Rivaroxaban is indicated for the treatment of deep vein thrombosis and pulmonary embolism, and prevention of recurrent deep vein thrombosis and pulmonary embolism in adults.
-
(S)-(+)-Glycidyl Phthalimide CAS 161596-47-0 Pu...
-
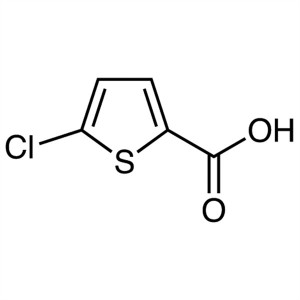
5-Chlorothiophene-2-Carboxylic Acid CAS 24065-3...
-
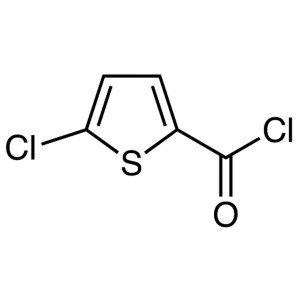
5-Chlorothiophene-2-Carbonyl Chloride CAS 42518...
-
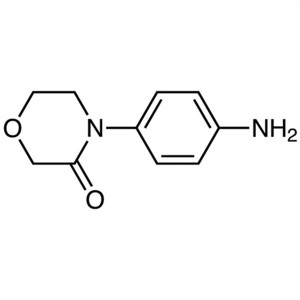
4-(4-Aminophenyl)morpholin-3-One CAS 438056-69-...
-
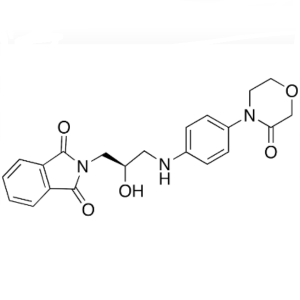
Rivaroxaban Intermediate CAS 446292-07-5 Purity...
-
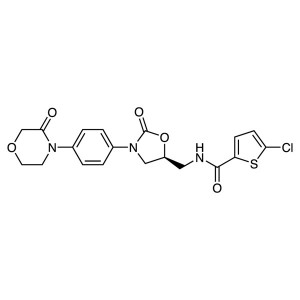
Rivaroxaban CAS 366789-02-8 Purity >99.5% (HPLC...
-
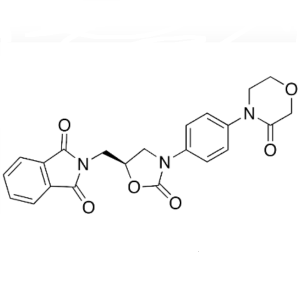
Rivaroxaban Intermediate CAS 446292-08-6 Purity...