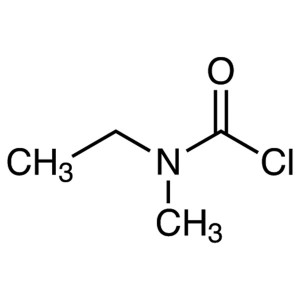
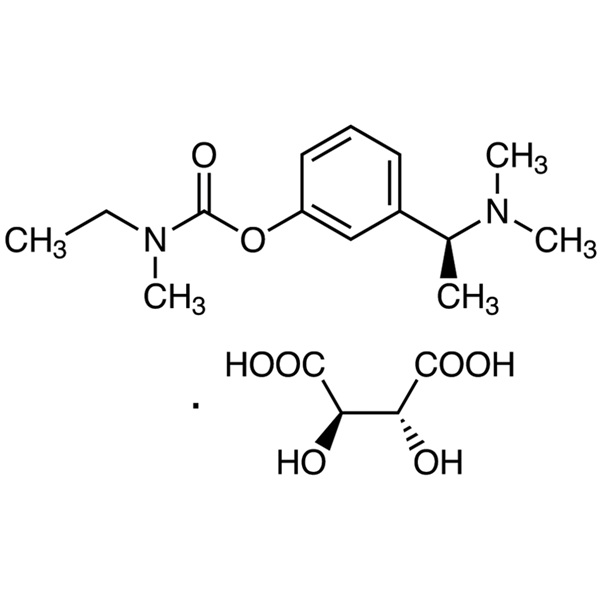
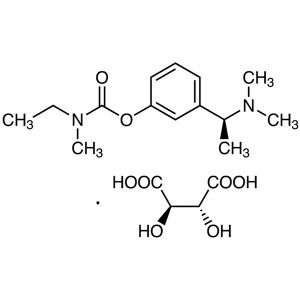
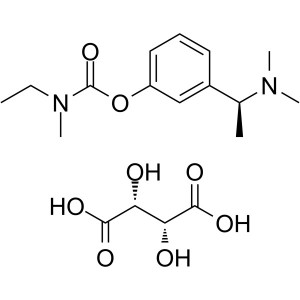
Rivastigmine Tartrate CAS 129101-54-8 Assay 98.0~102.0
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Rivastigmine Tartrate (CAS: 129101-54-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Rivastigmine Tartrate, Please contact: alvin@ruifuchem.com
| Chemical Name | Rivastigmine Tartrate |
| Synonyms | Exelon; ENA-713; Rivastigmine L-Tartrate; Rivastigmine Hydrogen Tartrate; CS-118; S-Rivastigmine Tartrate; 3-[(S)-1-(Dimethylamino)ethyl]phenyl N-Ethyl-N-Methylcarbamate L-Tartrate; N-Ethyl-N-Methylcarbamic Acid 3-[(S)-1-(Dimethylamino)ethyl]phenyl Ester L-Tartrate |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 129101-54-8 |
| Related CAS | 123441-03-2 |
| Molecular Formula | C14H22N2O2·C4H6O6 |
| Molecular Weight | 400.43 g/mol |
| Melting Point | 124.0 to 128.0℃ |
| Specific Rotation [a]20/D | +4.0° to +7.0° (C=5, Methanol) |
| Solubility | Soluble in Methanol |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Crystalline Powder | Complies |
| Assay | 98.0~102.0% (on the Anhydrous Basis) | 99.8% |
| Water by Karl Fischer | ≤0.50% | 0.15% |
| Residue on Ignition | ≤0.10% | 0.07% |
| Heavy Metals (Pb) | ≤20ppm | <10ppm |
| Phenol Impurity | ≤0.30% | <0.30% |
| DPTTA | ≤0.15% | <0.15% |
| Nor Impurity | ≤0.15% | <0.15% |
| Carbamate Impurity | ≤0.15% | <0.15% |
| Ether Impurity | ≤0.15% | <0.15% |
| Any Other Impurity | ≤0.10% | <0.10% |
| Total impurities | ≤0.50% | <0.50% |
| R-Enantiomer | ≤0.30% | <0.30% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the USP35 Standard | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Rivastigmine Tartrate
C14H22N2O2·C4H6O6 400.42
Ethylmethylcarbamic acid, 3-[(S)-1-(dimethylamino)ethyl]phenyl ester, (2R,3R)-2,3-dihydroxybutanedioate;
(S)-3-[1-(Dimethylamino)ethyl]phenyl ethylmethylcarbamate, hydrogen tartrate [129101-54-8].
Rivastigmine 250.34 [123441-03-2].
DEFINITION
Rivastigmine Tartrate contains NLT 98.0% and NMT 102.0% of the labeled amount of C14H22N2O2·C4H6O6, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption <197K>
• B. The retention time of the major peak of the Sample solution corresponds to that of the System suitability solution, as obtained in the test for Organic Impurities, Procedure 2: Enantiomeric Purity.
ASSAY
• Procedure
Buffer: 8.6 mg/mL of monobasic ammonium phosphate. Adjust with ammonia solution to a pH of 7.0.
Mobile phase: Methanol, acetonitrile, and Buffer (15:15:70)
System suitability solution: 0.05 mg/mL each of USP Rivastigmine Related Compound A RS and USP Rivastigmine Related Compound B RS in Mobile phase
Standard solution: 0.2 mg/mL of USP Rivastigmine Tartrate RS in Mobile phase
Sample solution: 0.2 mg/mL of Rivastigmine Tartrate in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 5-µm packing L7
Flow rate: 1.2 mL/min
Injection size: 20 µL
[Note-The flow rate may be adjusted to 1.5 mL/min, if needed, to achieve a recommended retention time of rivastigmine at about 10 min. ]
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between rivastigmine related compound A and rivastigmine related compound B, System suitability solution
Column efficiency: NLT 5000 theoretical plates, Standard solution
Tailing factor: NMT 3.0, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C14H22N2O2·C4H6O6 in the portion of Rivastigmine Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.1%
• Heavy Metals, Method II <231>: NMT 20 ppm
Organic Impurities
• Procedure 1
Mobile phase and System suitability solution: Proceed as directed in the Assay.
Standard solution: 1.0 µg/mL of USP Rivastigmine Tartrate RS in Mobile phase
Sample solution: 1.0 mg/mL of Rivastigmine Tartrate in Mobile phase
Chromatographic system: Proceed as directed in the Assay.
(See Chromatography <621>, System Suitability.)
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between rivastigmine related compound A and rivastigmine related compound B, System suitability solution
Relative standard deviation: NMT 10%, Standard solution
Analysis [Note-The run time is 8 times the retention time of the rivastigmine peak. ]
Samples: Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Rivastigmine Tartrate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response for each impurity from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Rivastigmine Tartrate RS in the Standard solution (mg/mL)
CU = concentration of Rivastigmine Tartrate in the Sample solution (mg/mL)
F = relative response factor (see Impurity Table 1)
Acceptance criteria
Individual impurities: See Impurity Table 1.
Total impurities: NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria NMT % |
| Tartrate | 0.18 | — | Disregard |
| Phenol impuritya | 0.28 | 1.6 | 0.3 |
| DPTTAb | 0.46 | 0.83 | 0.15 |
| Nor impurityc | 0.57 | 1.2 | 0.15 |
| Rivastigmine | 1.0 | 1.0 | — |
| Carbamate impurityd | 4.1 | 1.3 | 0.15 |
| Ether impuritye | 6.5 | 1.4 | 0.15 |
| Any other impurity | — | 1.0 | 0.1 |
a (S)-3-[1-(Dimethylamino)ethyl]phenol.
b (+)-Di-(p-toluoyl)-d-tartaric acid (rivastigmine related compound A).
c (S)-3-[1-(Dimethylamino)ethyl]phenyl dimethylcarbamate (rivastigmine related compound B).
d 3-Nitrophenyl ethyl(methyl)carbamate.
e (S)-N,N-Dimethyl-1-[3-(4-nitrophenoxy)phenyl]ethanamine.
• Procedure 2: Enantiomeric Purity
Buffer: Transfer 1.78 g of dibasic sodium phosphate dihydrate and 1.38 g of monobasic sodium phosphate into a 1000-mL volumetric flask. Dissolve in and dilute with water to volume. Adjust with phosphoric acid to a pH of 6.0.
Mobile phase: Transfer 20 mL of acetonitrile and 205 µL of N,N-dimethyloctylamine to a 1000-mL volumetric flask, and dilute with Buffer to volume.
Standard solution: 0.1 µg/mL of USP Rivastigmine Tartrate R-Isomer RS in Mobile phase
Sensitivity solution: 0.05 µg/mL of USP Rivastigmine Tartrate R-Isomer RS in Mobile phase, Standard solution
System suitability solution: 100 µg/mL of USP Rivastigmine Tartrate RS and 0.1 µg/mL of USP Rivastigmine Tartrate R-Isomer RS in Mobile phase
Sample solution: 100 µg/mL of Rivastigmine Tartrate in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.0-mm × 10-cm; packing L41
Flow rate: 0.5 mL/min
Injection size: 20 µL
System suitability
Samples: Standard solution, Sensitivity solution, and System suitability solution
Suitability requirements
Resolution: NLT 0.8 between the enantiomer peaks, System suitability solution
[Note-The elution order is the R-enantiomer, followed by the rivastigmine peak, which is the S-enantiomer. ]
Signal-to-noise ratio: NLT 10, Sensitivity solution
Relative standard deviation: NMT 10%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the R-enantiomer in the portion of Rivastigmine Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of R-enantiomer from the Sample solution
rS = peak response of R-enantiomer from the Standard solution
CS = concentration of R-enantiomer in the Standard solution (µg/mL)
CU = concentration of Rivastigmine Tartrate in the Sample solution (µg/mL)
Acceptance criteria: NMT 0.3% of the R-enantiomer
SPECIFIC TESTS
• Water Determination, Method Ia <921>: NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers, and store at room temperature.
• USP Reference Standards <11>
USP Rivastigmine Tartrate RS
USP Rivastigmine Related Compound A RS
Di-p-toluoyl-d-(+)-tartaric acid monohydrate.
C20H20O9 404.37
USP Rivastigmine Related Compound B RS
N,N-Dimethylcarbamic acid-3-[1-(dimethylamino)ethyl]phenyl ester.
C13H20N2O2 236.32
USP Rivastigmine Tartrate R-Isomer RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
UN IDs UN 2811 6.1 / PGII
WGK Germany 3
RTECS FA9550000
HS Code 29242990
Hazard Class 6.1
Packing Group III
Rivastigmine Tartrate (CAS: 129101-54-8) is the tartrate of rivastigmine, a drug for the treatment of Alzheimer's disease. Rivastigmine is a physostigmine derivative, which was successfully developed for the first time by Novartis, Switzerland. The trade name is exelon, and the molecule has a benzyl carbamate structure, it is a carbamate brain-selective cholinesterase inhibitor, which can inhibit acetylcholinesterase and butyrylcholinesterase at the same time, and promote cholinergic nerve conduction by delaying the degradation of acetylcholine released by cholinergic neurons. It can improve the cognitive dysfunction mediated by cholinergic, thereby improving the cognitive effect of patients with Alzheimer's disease. The plasma protein binding capacity of rivastigmine is weak, easy to pass through the blood-brain barrier, and has a high degree of brain selectivity. It can not only selectively act on the most vulnerable areas of the cerebral cortex and hippocampus, but also preferentially inhibit the dominant subtypes of AChE in the brain, which can reduce peripheral cholinergic side effects while producing curative effects. The half-life of rivastigmine tartrate in the body is short and the action time is long. Unlike tacrine, this product has a stronger inhibitory effect on G1 enzyme in the hippocampus and cortex. It is clinically used to treat mild to moderate Alzheimer's dementia, which is suspected of Alzheimer's disease or Alzheimer's disease.
1. As an acetylcholinesterase inhibitor, rivastigmine bicartrate can improve the effect of succinylcholine muscle relaxant. Therefore, there should be a suitable intermittent period to stop taking this product before anesthesia. This product should be combined with other cholinergic or anticholinergic preparations, and caution should be taken (see [Drug Interaction]).
2. Due to their pharmacological effects, cholinesterase inhibitors may have vagus nerve tension effects on heart rate. As with other cholinergic drugs, caution must be exercised when it is given to patients with sick sinus syndrome or other heart block (see Adverse Reactions).
3. Cholinergic nerve excitement can cause increased gastric acid secretion. Although no evidence of significant deterioration of the corresponding symptoms was found during the clinical trial period, patients with a high risk of gastric ulcer, such as those with a history of ulcer disease or those receiving concomitant treatment with non-steroidal anti-inflammatory drugs, should be used with caution.
4. Like other cholinesterase inhibitors, patients with a history of asthma or other obstructive pulmonary disease should be used with caution.

![(S)-3-[1-(Dimethylamino)ethyl]phenol CAS 139306-10-8 Rivastigmine Tartrate Intermediate Purity >99.0% (GC)](https://www.ruifuchem.com/uploads/S-3-1-Dimethylaminoethylphenol-CAS-139306-10-8-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
![3-[1-(Dimethylamino)ethyl]phenol CAS 105601-04-5 Rivastigmine Tartrate Intermediate Purity >98.0% (HPLC)](https://www.ruifuchem.com/uploads/3-1-Dimethylaminoethylphenol-CAS-105601-04-5-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)